How organic molecules can arise in space
Researchers could find a mechanism that would explain the formation of complex organic molecules in space: In laboratory experiments, they encountered unusual icy properties, which must also be demonstrated by ice-covered dust grains that enable chemical reactions in space. However, further experiments are necessary to confirm this.
|
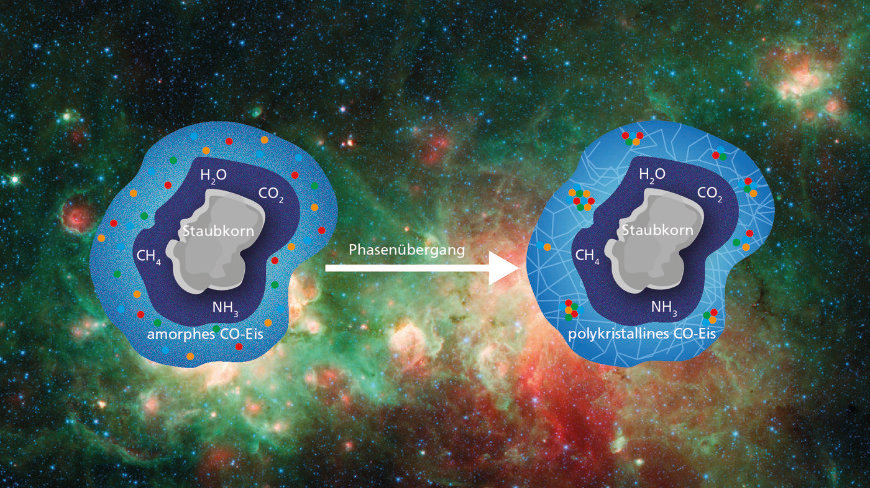
In the early stages of star formation, ice is likely to undergo a phase transition on cosmic dust grains (amorphous to polycrystalline ice). This phase transition appears to help the molecules, or radicals, inside the ice to stick together. In the resulting “clusters of molecules”, chemical reactions can form more complex organic molecules. picture: NASA/JPL-Caltech, MPIA Graphic Design [Großansicht] |
Laboratory experiments simulating how layers of ice form on tiny cosmic grains of dust have provided a possible answer to one of the most open questions in astrochemistry: how complex organic molecules can form in interstellar molecular clouds. These particles, carried to Earth by meteorites, could be one of the ingredients that gave rise to life on Earth and possibly other suitable planets.
The vast interstellar spaces are nearly empty, but not completely—and in the thin clouds of gas and dust that can be found there, astronomers have discovered more and more complex molecules in recent decades. Interestingly, a number of about 200 interstellar molecular species known in the interstellar space are organic – these are the names in particular for the more complex carbon-containing molecules. Here on Earth, these molecules are the basis of life as we know it.
Unsurprisingly, this led to the question whether interstellar organic molecules could be linked in any way to the origin of life on Earth and perhaps also life on other planets. One possible scenario envisions that meteorites transport interstellar organic molecules into small pools here on Earth, thus creating the conditions for life to arise.
But how can complex organic molecules form in interstellar space? The interstellar molecular clouds there are of very low density. Even the densest of these clouds, with about a hundred thousand gas molecules per cubic centimeter, corresponds to what engineers at least call a “super vacuum” when trying to mimic such conditions here on Earth. Ordinary chemical reactions, in which molecules or atoms meet and then combine with each other, occur very rarely under these conditions to produce anything other than very simple molecules.
In the 1960s, researchers interested in interstellar chemistry began developing the idea that grains of interstellar dust could serve as “cosmic laboratories” in which more complex chemical reactions would take place. Such grains of dust, which are based on carbon or silicate and are less than a millionth of a meter in diameter, typically form in the outer layers of cold stars or as a result of supernova explosions. In the interstellar molecular cloud, these dust grains accumulate an outer layer of water ice, and these ice layers will in turn serve as little cosmic chemical laboratories.
The ice that encases a cosmic particle of dust usually has an onion-like structure with dozens of successive layers. The inner layers are mainly composed of water ice, but they also contain molecules such as carbon dioxide, ammonia, methane, and others. Chemically, the outer layers are more interesting. The main ingredient here is carbon monoxide (CO) ice, mixed with other ingredients such as methanol or formaldehyde organic compounds. It can also contain hydrogen and oxygen atoms, as well as compounds called “radicals” that are particularly avid in chemical reactions: hydroxyl groups, formyl radicals, methoxy groups, hydroxymethyl groups, and others.
Previous laboratory experiments have conclusively demonstrated that chemical reactions between these reactive species in CO-rich layers of ice lead to the formation of many interesting complex organic molecules, including methyl formate, glycolaldehyde and ethylene glycol – even at temperatures as low as 10 K, which are Typical in interstellar molecular clouds. However, the big open question was “how” it all. In order to form more complex molecules, the chemicals in the ice must move to the same place. After all, chemical reactions cannot occur at a distance. Previous models have had difficulties here—and new work by she and his colleagues offers a possible solution to this problem.
The particles in solid ice are not completely frozen in place. In any piece of non-zero-temperature material, the atoms constantly vibrate slightly, and this oscillation allows the embedded molecules to change position—either by “squeezing” through it or because one of the myriad bonds that hold the ice together is temporarily loosened or even broken. This kind of random movement is called diffusion.
An important task of laboratory astrochemistry is to determine the diffusion rates of various atoms, molecules, and radicals on and within the ice sheet of a piece of dust. However, the results for solid ice are discouraging: except for small hydrogen atoms and molecules, diffusion in ice is very slow at 10 K, the typical temperature of interstellar molecular clouds. This is a big problem for the formation of more complex molecules. Unless the starting materials sit next to each other by chance, the necessary chemical reactions simply do not occur in such conditions.
This was the situation when Francis Torrillo, then a doctoral student at
Syracuse University, his supervisors Jiao He (initially at Leiden University, later at the Max Planck Institute for Astronomy) and Gianfranco Vidali (Astrophysics and Surface Science Laboratory In the Syracuse University) The formation of carbon dioxide ice layers on dust grains began to be investigated closely. According to a plan developed by He, Torrillo devised a very high vacuum environment in which he placed a small 13 mm gold-plated copper disc. The disk is supposed to represent the surface of a cosmic speck of dust. It is connected to an external cooling device and can be cooled in a controlled manner at temperatures up to 5 K.
By introducing water vapor or carbon dioxide gas into the chamber, researchers can systematically grow layers of water ice or carbon dioxide ice on the disk. Then the ice layers are observed with an infrared spectrophotometer: an infrared light lamp is illuminated on the ice and the reflected light is analyzed. The way the material absorbs light at certain wavelengths provides information about the properties of ice. A Crucial Phase In a series of experiments, the researchers first prepared a multi-layered water-ice “core” and then placed layers of carbon monoxide ice of varying thicknesses on top of it at 6 K. Then they heated the sample to 20 K and carefully monitored the infrared spectra throughout.
At about 10 K, a typical temperature in dense interstellar clouds, something interesting happened: the infrared spectrum shifted in a way that the researchers interpreted as a phase transition. Below this temperature, the carbon monoxide ice was in an amorphous phase where carbon dioxide molecules stick together in all directions. Above this temperature, the phase changes, possibly to the so-called polycrystalline phase: a collection of many small carbon dioxide ice crystals.
To find out what this means for carbon dioxide ice’s role as a “cosmic laboratory,” the researchers created a second version of the experiment in which carbon dioxide (CO) was used.2 It was added when the first layers of carbon dioxide ice formed. CO . company2
It should generally refer to any kind of additional chemicals that might stick to the ice layer of cosmic dust. Below 10 K everything was as expected: carbon monoxide2The molecules were individually suspended in the ice and were not able to assemble and thus potentially participate in chemical reactions. But during the time when the phase transition took place, the situation changed radically. Next, the infrared spectrometer showed a strong signal of CO clusters2Molecules that obviously found each other and clump together. During the transition to the polycrystalline form of carbon dioxide ice, carbon dioxide2Molecules and possibly radicals and other molecules seem to move around in the ice and in this way create suitable starting conditions for chemical reactions.
Based on their experimental results, the researchers made more general calculations about what the phase transition could mean for ice-capped dust grains in massive interstellar clouds. They came to the conclusion that the phase transition to the polycrystalline state in such clouds should be the norm: in the early stages of star formation, when parts of the cloud begin to collapse and thus heat up. The carbon dioxide ice on the dust grains in the region should become polycrystalline. During this transition, radicals and molecules can migrate more freely than normal and form larger groups of molecules.
If someone extrapolates from carbon monoxide2 On more complex particles or on roots, this could explain the efficiency of cosmic chemistry labs based on dust grains: Over time, grains of cosmic dust will collect ice as well as roots or particles. Once star formation begins, the phase transition will ensure that many of these radicals and molecules can fuse together. This would create the conditions under which chemical reactions could occur and more complex molecules could arise—molecules that might play a role in creating new life after being transported to a newly formed planet by meteorites. In light of the experiments conducted so far, this is a fascinating and attractive scenario.
It is possible that this kind of “operational sequencing” of cosmic chemistry laboratories is of great importance in the formation of complex organic molecules and, ultimately, life. But there is still a long way to go before the evidence is strong enough for the scenario to be widely accepted in the scientific community. Then, he, who has meanwhile moved to Heidelberg as head of the newly established MPIA “Origins of Life” laboratory, plans a version of the experiment with his colleagues in which radicals and other molecules are used as carbon monoxide.2
Subject to transition. If this results in the same aggregation effect, it would be another step toward defining the role of the transition into polycrystalline carbon dioxide ice in the astrochemistry of interstellar clouds.
The team reports the results in a specialized article in the journal Astrophysical Journal Letters He appeared.
|
|
|
|
|
|

“Social media evangelist. Baconaholic. Devoted reader. Twitter scholar. Avid coffee trailblazer.”