Neither solid nor liquid: Physicists have discovered that water can take on new states under confined conditions. In accordance with this, a single layer of water molecules can already transform into a “hexagonal” phase at room temperature – it is neither solid nor liquid and H2O molecules spin into place. When the pressure is slightly increased, this water becomes super-ionic, assuming a state previously assumed only for planetary cores, as the physicists reported in Nature.
Water every day and strange at the same time. Because the seemingly simple H2O molecule actually shows some properties under normal conditions – from Density abnormality about self disintegration into more than ten different ice shapes. However, it becomes even more peculiar if the water molecules are tightly bound, for example in narrow capillaries: then the water suddenly loses its dipole moment and becomes “Electrically dead”“, In addition to Float Suddenly with less resistance than it should be.
Trapped water everywhere
And that’s not all, physicists led by Venkat Kapil of the University of Cambridge have now discovered. Using complex physical models and artificial intelligence, they investigated how the structure of a single layer of water changes under different temperature and pressure conditions. “Water in such nanocavities is ubiquitous and plays a key role in a myriad of everyday phenomena,” the team explains.
These very thin layers of water occur, for example, in the capillaries and membranes of all living things, make up the pore water in the rocks of the Earth’s interior and play an important role in many technical, medical and chemical applications. However, little is known about the behavior of ‘tight’ water under different conditions. The only thing that is clear is that water molecules are under high pressure in such narrow environments due to the gravitational force of the walls.
Phase Diagram for Single Layer Water
The Kapil team has now succeeded in creating a phase diagram for a single layer of water – a diagram showing changes as a function of temperature and pressure. Their modeling represents the states of single-layer waters for the first time according to the first-principles method, this is based only on known regularities and thus ensures that the results are not falsified with assumptions and hypotheses. This method is considered the gold standard for physical computations and modeling.
The analyzes initially revealed some well-known phase transitions: at temperatures below freezing point, monolayer water goes through different icy phases. With increasing pressure, the arrangement of the water molecules changes from a regular hexagonal atomic lattice to a pentagonal to a square and finally to a flat rhombic crystal lattice. Water passes through several triple points, in each phase there are three phases at the same time, physicists say.
Hexagonal phase: neither solid nor liquid
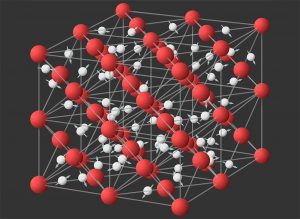
But then a surprising thing happened: at room temperature and a pressure of 0.5 to 2 GPa, which is typical for nanochannels, the water changed into a strange state that had not been observed before. “Water goes through a phase that is neither solid nor liquid,” Capel and colleagues explain. The atoms of the water molecules are still arranged roughly in a hexagonal pattern, but the molecules are constantly spinning around on themselves.
Such a state of water would only have been hypothesized by a few physicists. This peculiar combination of hexagonal symmetry and strong rotation is referred to as the hexagonal phase. The analyzes conducted by Cappel and his team now indicate for the first time that this hexagonal state does indeed occur in nanochannels at room temperature – and thus can be verified experimentally.
Superionic even at moderate pressure
But the analyzes had another surprise in store: at about 75 degrees and a pressure of four gigapascals, monolayer water changed its state again – it became super-ionic. In such an ionic super-state, the oxygen atoms remain localized and form a kind of lattice, while hydrogen protons migrate freely through this lattice. This gives the water increased conductivity.
The amazing thing about it: Ordinary, unconstrained water would need more than 420 degrees and about 56 gigapascals to become above ionic – this corresponds to pressure deep underground. “The existence of a super-ionic phase under conditions that are relatively easy to produce is interesting because it usually only occurs under extreme conditions, such as in the cores of the planets Uranus and Neptune,” Cappel explains. also material Earth’s inner core It could be super-ionic, researchers recently reported.
Also important for practical applications
This study thus confirms the extent to which the properties of materials change when they are confined to one or a few molecular layers. For water, it also reveals the presence of several strange phases and a completely new state in the form of hexagonal water. The fact that hexagonal and ionic water occurs in narrow channels even under relatively normal conditions opens up entirely new possibilities for experimental investigations, physicists explain.
But above all, super-ionic water can also have very practical uses: in the super-ionic phase, water has a conductivity 100 to 1,000 times higher than common battery electrolytes. If this state could now be induced by gentle pressure in the nanotubes or between layers of graphene, it could make even more powerful batteries possible. The results also indicate that other materials also become superionized earlier than previously thought under restricted conditions. (Nature, 2022; doi: 10.1038/s41586-022-05036-x)
Source: Cambridge University

“Total coffee aficionado. Travel buff. Music ninja. Bacon nerd. Beeraholic.”






More Stories
Researchers detect extremely high-energy gamma rays
Anxiety disorders in old age increase the risk of dementia
Researchers are particularly fascinated by these exoplanets.